How to Balance Chemical Equations With Parentheses
Elementary Differential Equations William E. Group 17 elements are 1-.

Balancing Chemical Reactions With Parentheses Youtube
To make your equations more compact you may use the solidus the exp function or appropriate exponents.

. For example the earlier reaction could be written. Notation for a Chemical Equation. This requires a ratio of one Ca 2 ion to two H 2 PO 4 H 2 PO 4 ions.
All types of parentheses are correct for example K3FeCN6 To enter charge species just type them as they are for example Hg2 Hg22 or Hg22. Balancing for mass produces the same. They are most commonly used in redox reactions double replacement reactions and acid-base neutralisations.
Full PDF Package Download Full PDF Package. Balancing the molecular equation transforming to a complete. The state of matter of each compound or molecule is indicated in subscript next to the compound by an abbreviation in parentheses.
Tables I-1 and I-2 provide volumes in drops dp milliliters mL teaspoons tsp tablespoons tbls cups cp liters L and gallons gal of liquid bleach dry calcium hypochlorite HTH and a concentrated calcium hypochlorite solution that when added to the indicated volume of water will provide the approximate chlorine dose indicated. Net ionic equations are an important aspect of chemistry as they represent only the entities that change in a chemical reaction. 2 Fe 2 O 3 s 3 Cs 4 Fes 3 CO 2 g where s indicates a solid and g is a gas.
Save time and effort with authoring tools and resources that will help. How do you know if a chemical equation is a solid liquid or gas. Balancing another combustion reaction.
Enter an equation of a chemical reaction and click Submit for example. Balancing chemical equations 1. This is listed in parentheses immediately following the compound.
Download Full PDF Package. Remember when working with polyatomic ions use the charge of the complete polyatomic ion rather than the individual ions. Transition elements will have Roman numerals in parentheses to indicate their charge.
Preparing your work for publication with IEEE should be seamless. Example of Balanced Ionic Equation. CH 4 g 2 O 2 g CO 2 g 2 H 2 O g Stoichiometry Anatomy of a Chemical Equation Products appear on the right side of the equation.
Equation numbers within parentheses are to position flush right as in 1 using a right tab stop. For example a compound in the gas state would be indicated by g solid s liquid l and aqueous aq. Rules for typing equations.
Silver is 1 zinc is 2 and aluminum is 3. Group 16 elements are 2-. Group 15 elements are 3-.
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products and shows the direction of the reaction. So here we have a chemical equation. There are three basic steps to writing a net ionic equation.
Use a long dash rather. We designate this by enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript 2. Balance the positive and.
Steps to Use the Combine Like Terms Calculator. Italicize Roman symbols for quantities and variables but not Greek symbols. Video transcript - Voiceover Lets now see if we can balance a chemical equation with slightly more complex molecules.
28 Full PDFs related to this paper. Balancing chemical equation with substitution. If ions are present the sum of the positive and negative charges on both sides of the arrow is also the same.
In aqueous solutions its common to balance chemical equations for both mass and charge. Spaces are irrelevant for example Cu SO 4 is equal CuSO4. An unbalanced equation lists the reactants and products but not the ratio between them.
CH 4 g 2 O 2 g CO 2 g 2 H 2 O g Stoichiometry Anatomy of a Chemical Equation The states of the reactants and products are written in parentheses to. A short summary of this paper. Thus we must have two negative charges to balance the 2 charge of the calcium ion.
The formula is CaH 2 PO 4 2. A balanced chemical equation has the same number and types of atoms on both sides of the arrow. Chemical equations may be either unbalanced or balanced.
This is a handy tool while solving polynomial equation problems as it makes the calculations process easy and quick. Anatomy of a Chemical Equation Reactants appear on the left side of the equation. Visually understanding balancing chemical equations.
Combine like terms calculator is a free online tool which can help to combine like terms in an equation and simplify the equation.
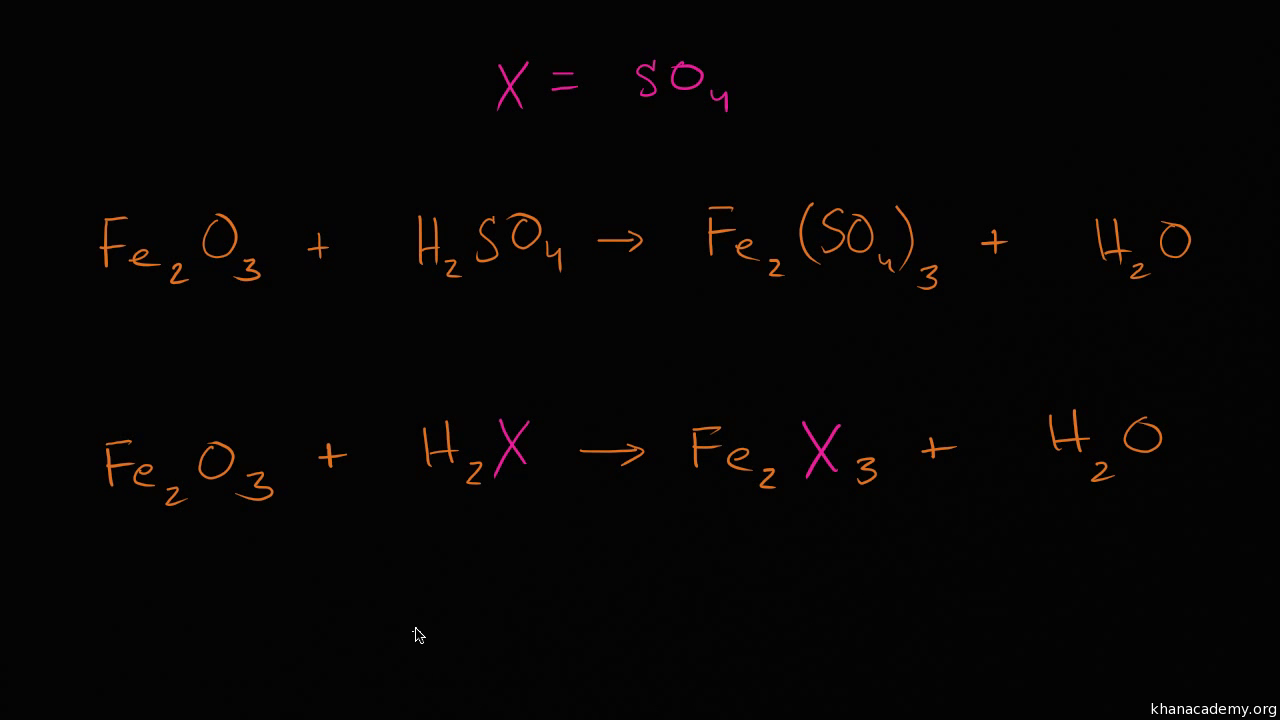
Balancing Chemical Equation With Substitution Video Khan Academy
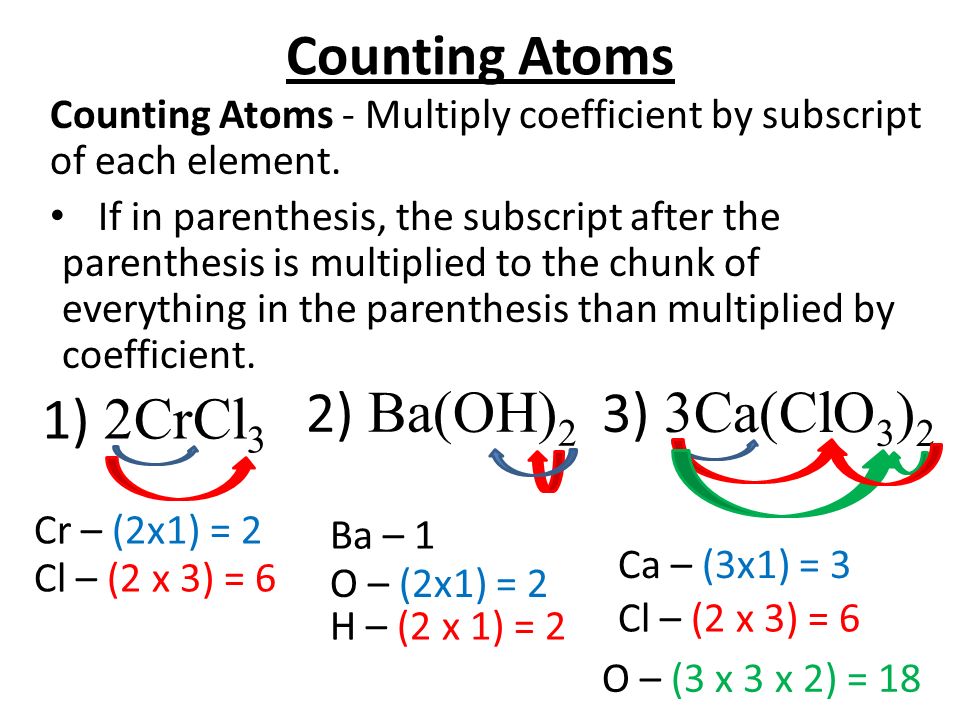
Counting Atoms Balancing Equations Law Of Conservation Of Mass Ppt Video Online Download
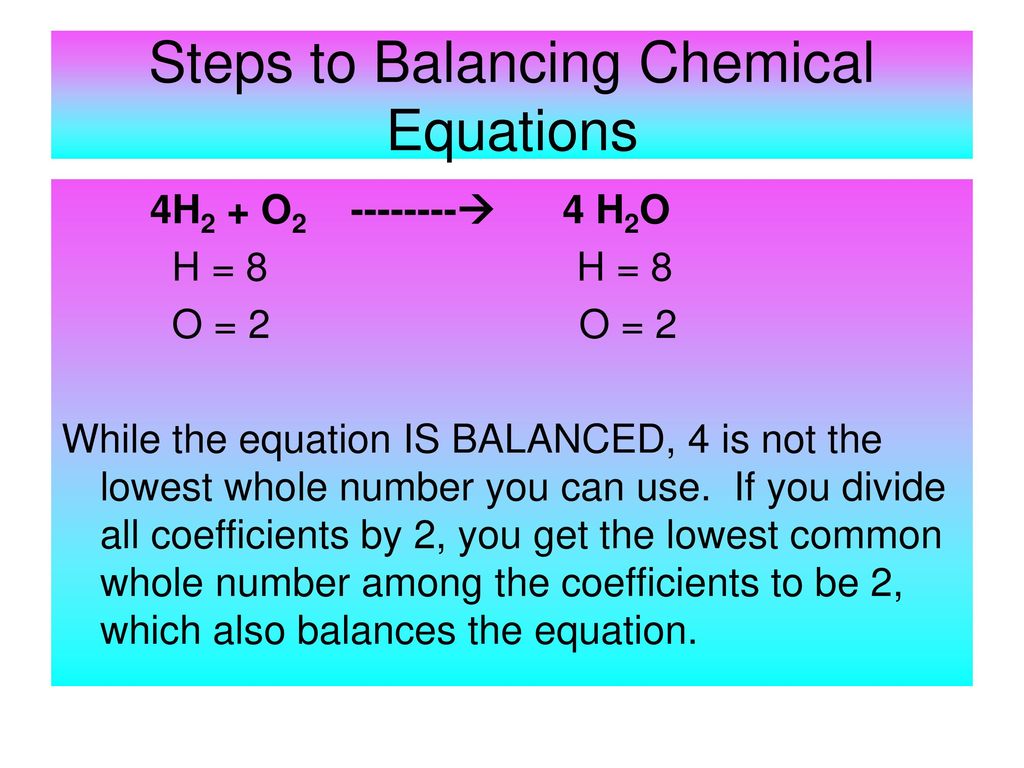
Chemical Reactions And Balancing Chemical Equations Ppt Download
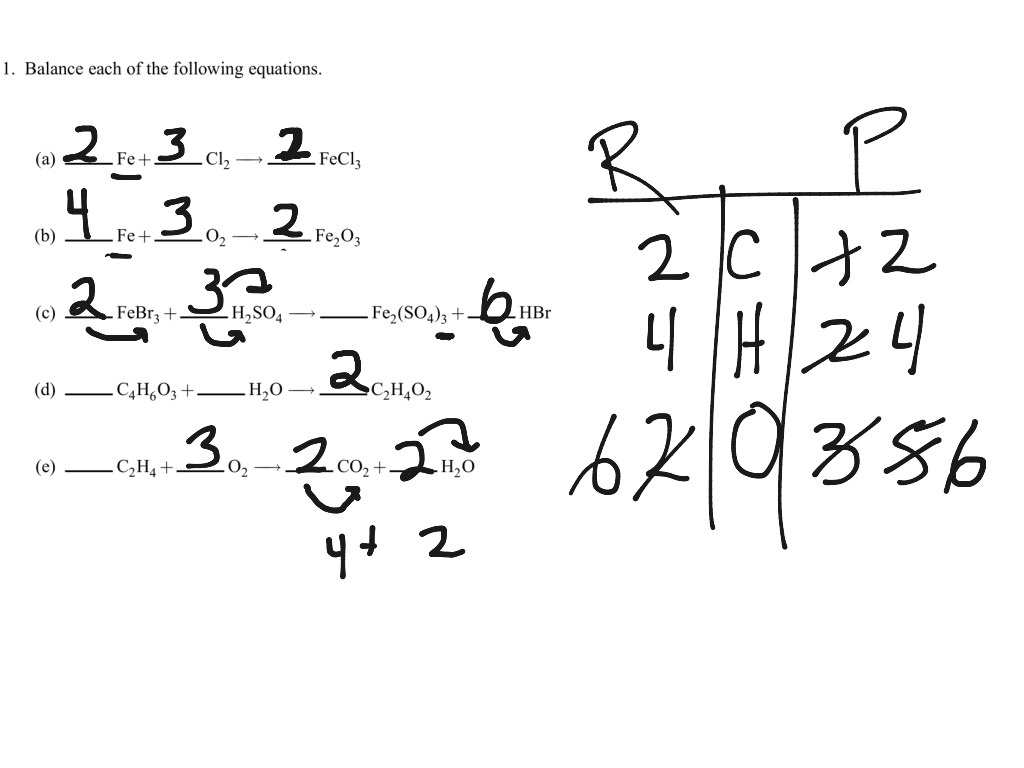
7 1 Balancing Equations Science Chemistry Balancing Equations Showme
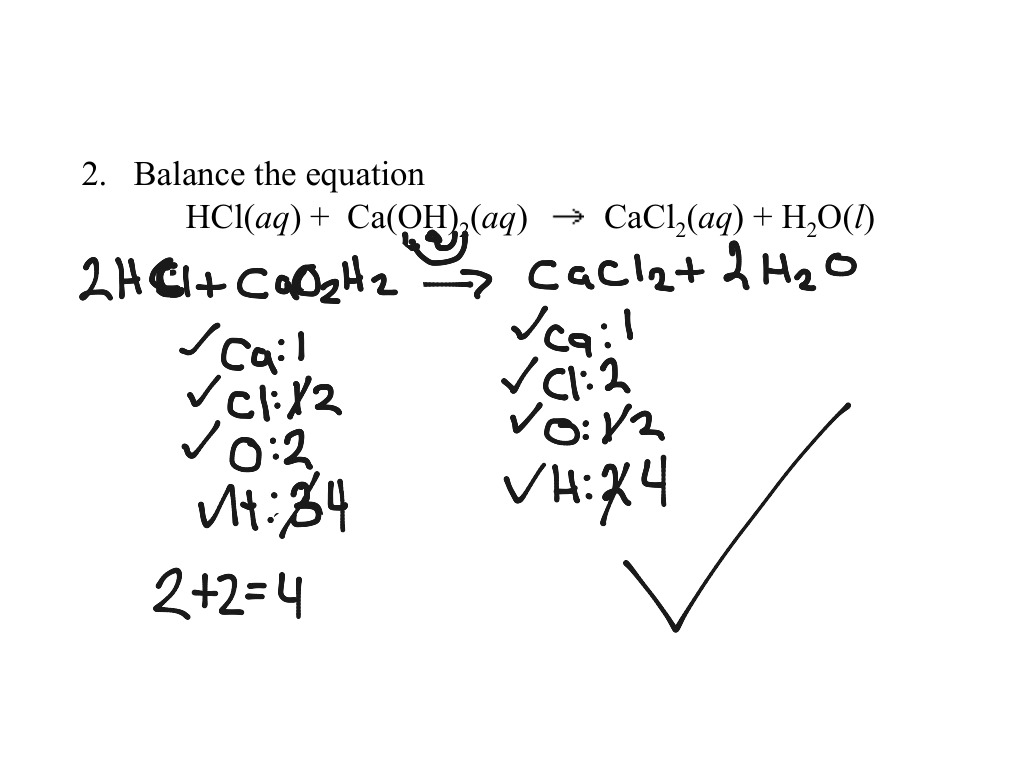
Balancing Equation With Parenthesis Science Chemistry Showme
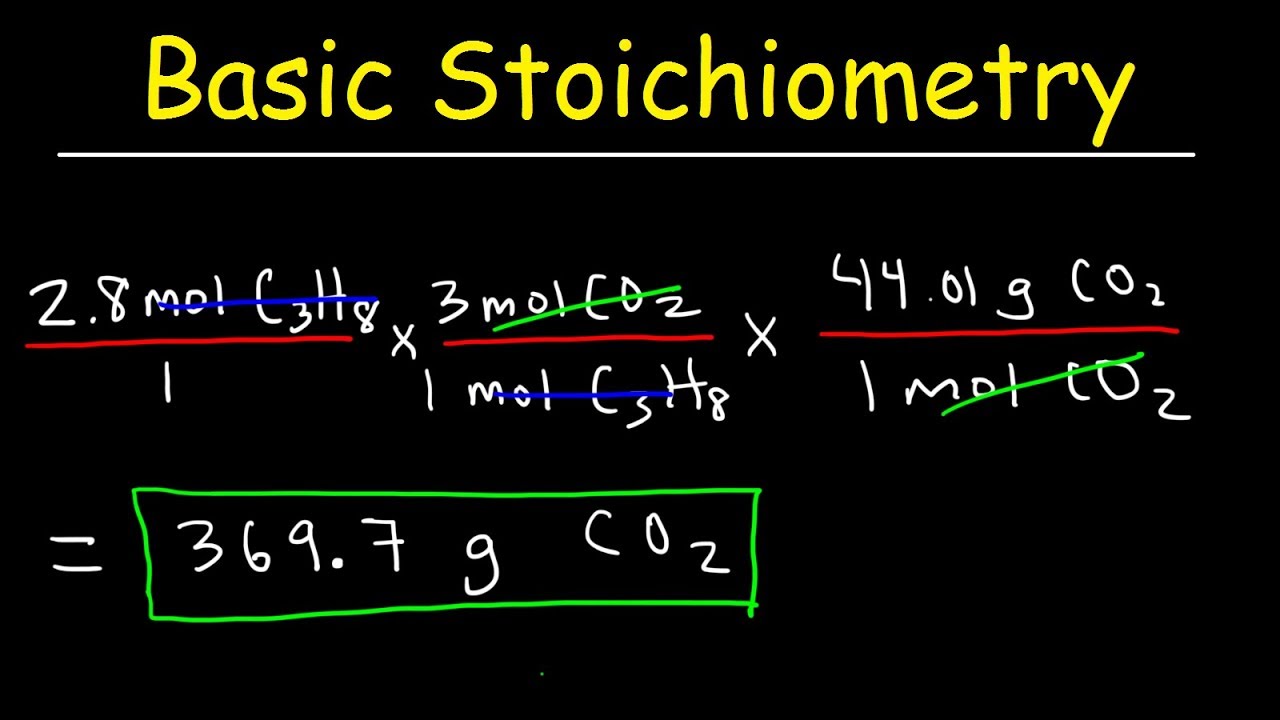
How To Balance Chemical Equations Youtube

How To Balance Chemical Equations Best Examples Get Education Bee

How To Balance Chemical Equations Youtube

Balancing Chemical Equations Questions A Process

Conservation Of Mass Balancing Chemical Equations 1 19 Ppt Download
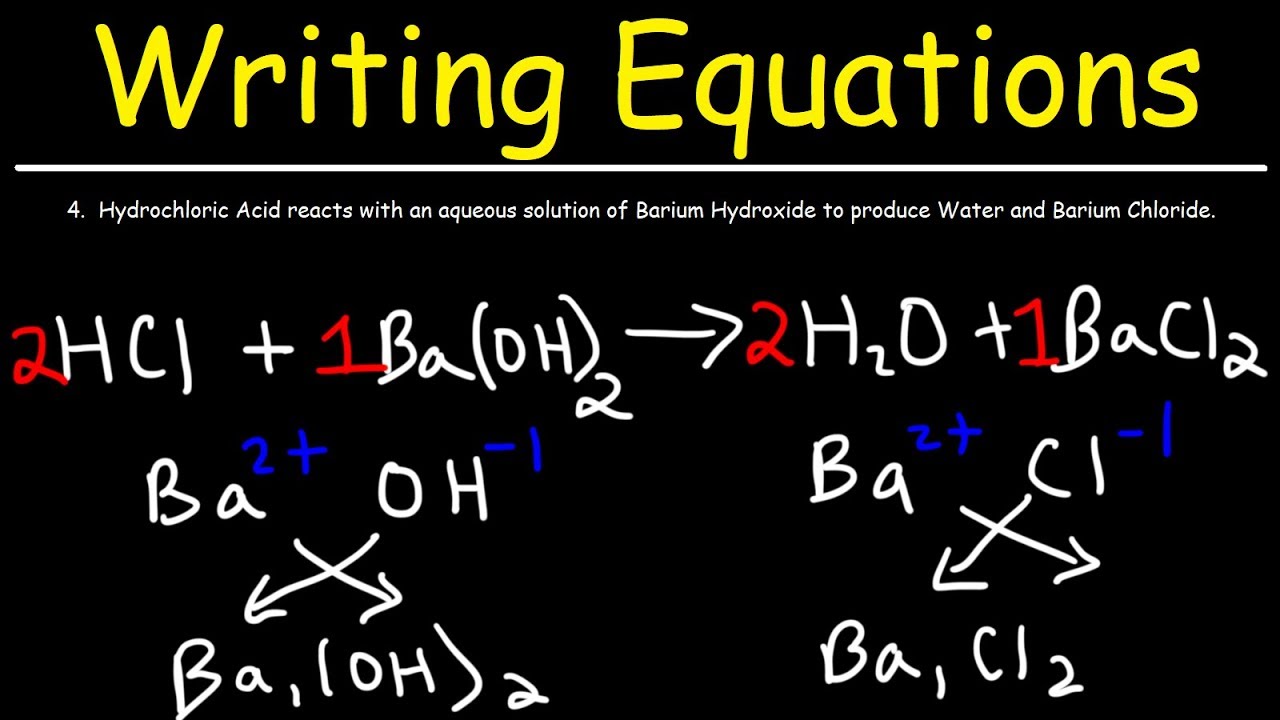
How To Write A Chemical Equation With Pictures Wikihow

How To Balance Chemical Equations Youtube
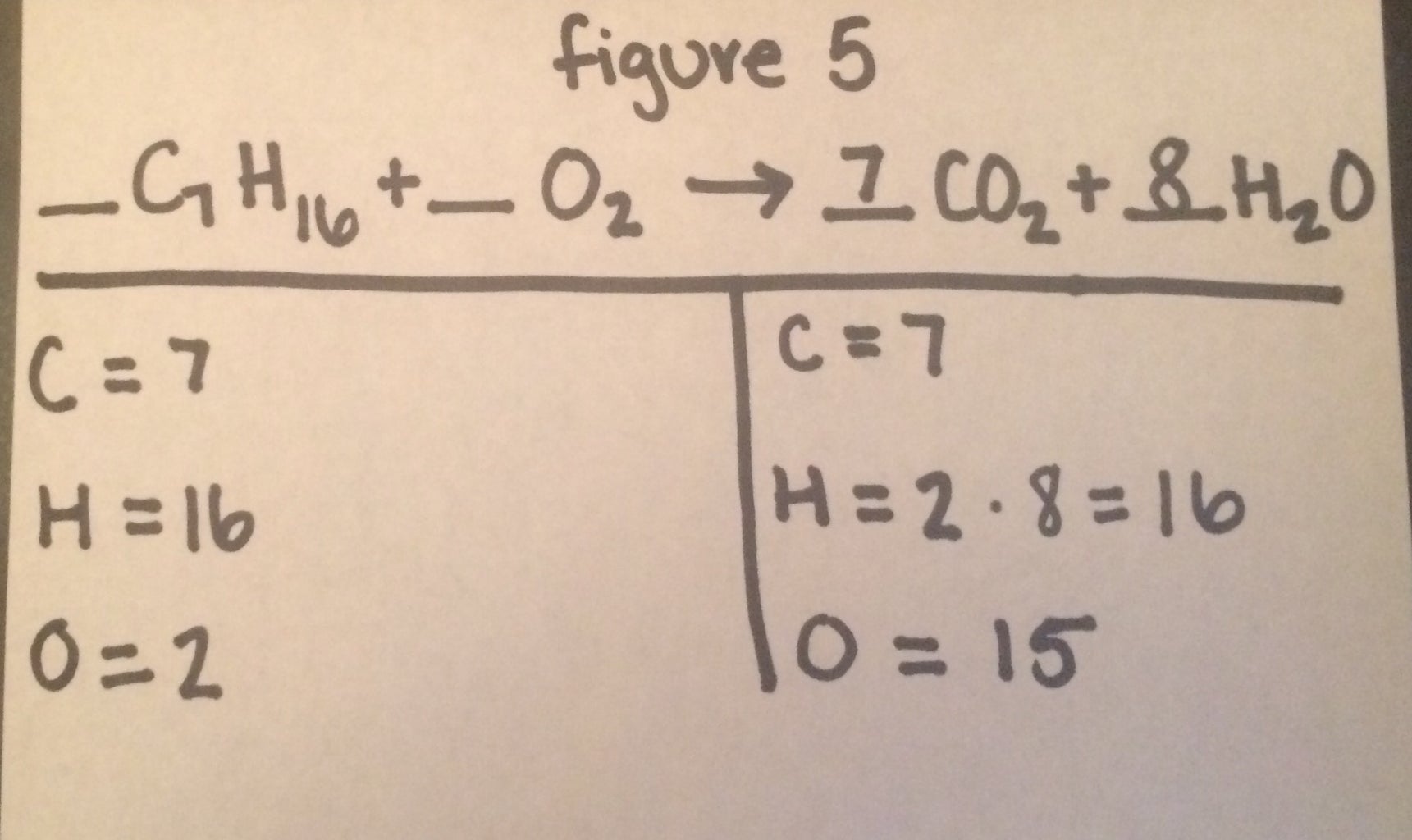
How To Balance A Chemical Equation Final 4 Steps With Pictures Instructables





Comments
Post a Comment